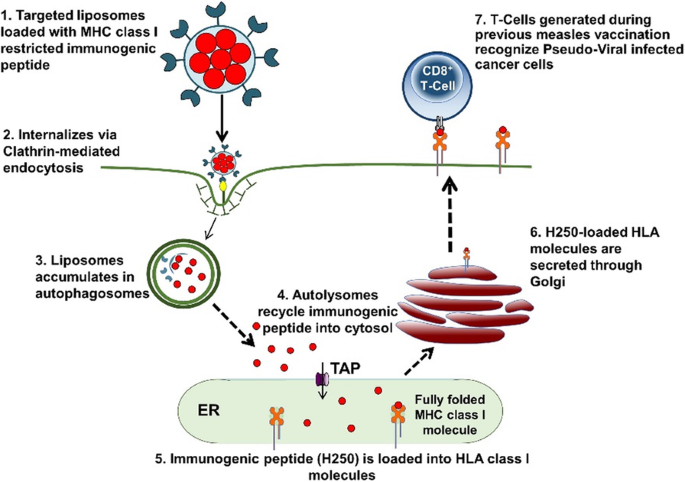
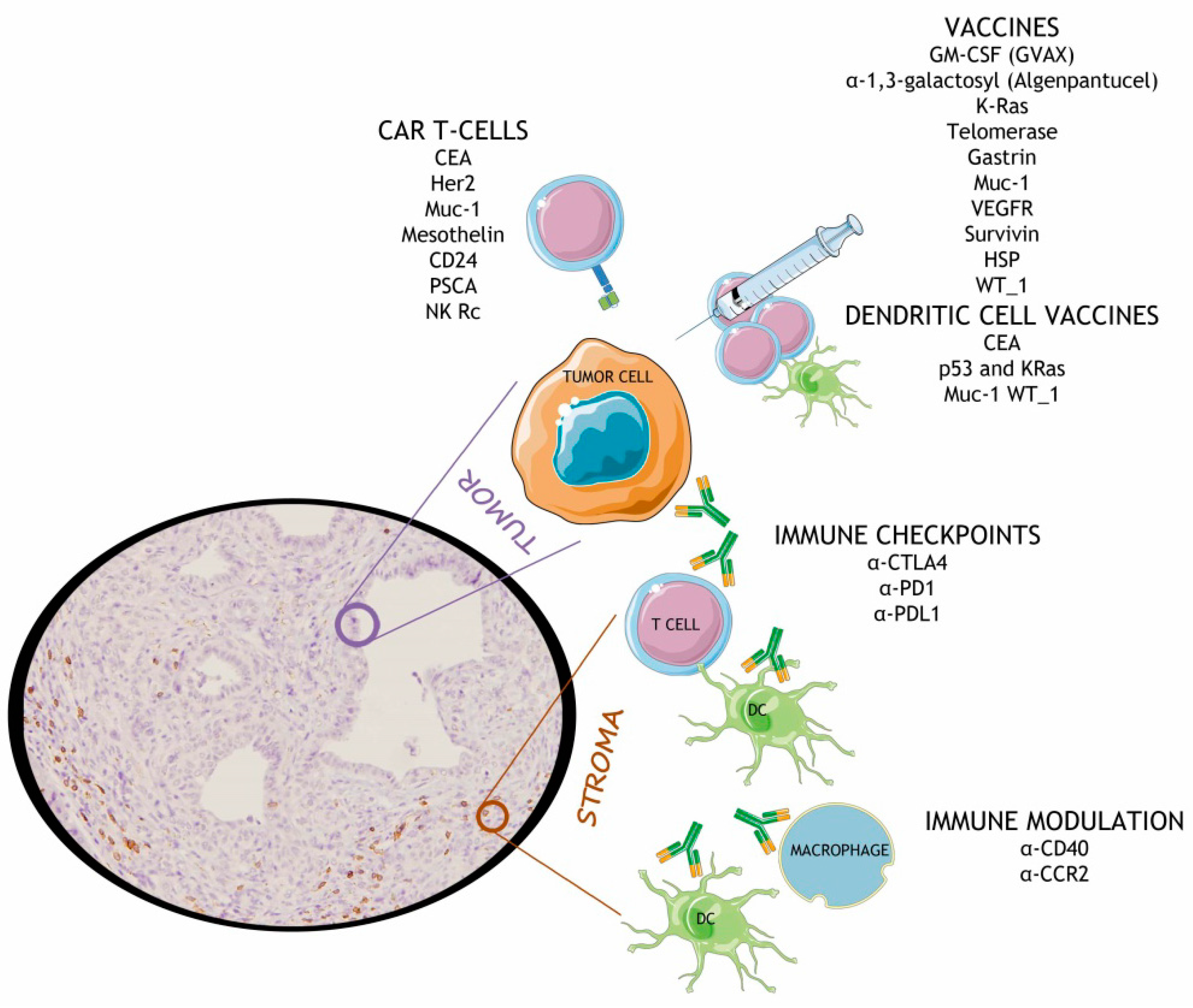
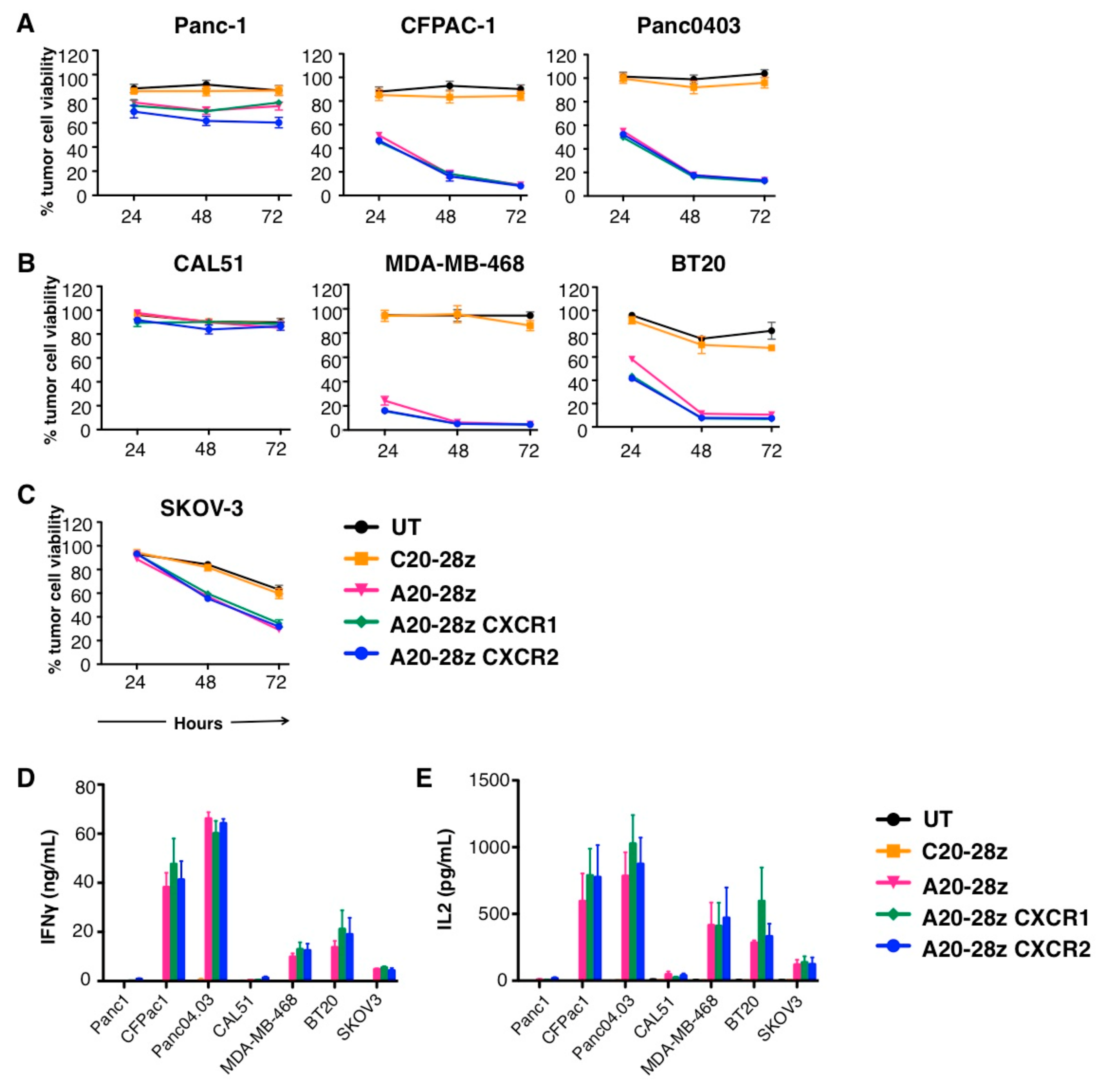
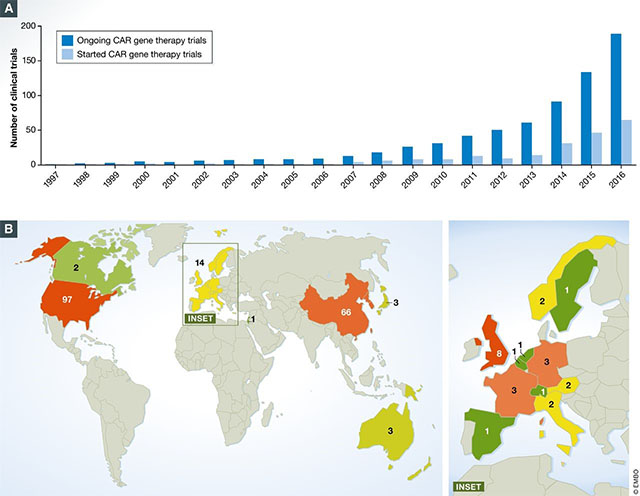
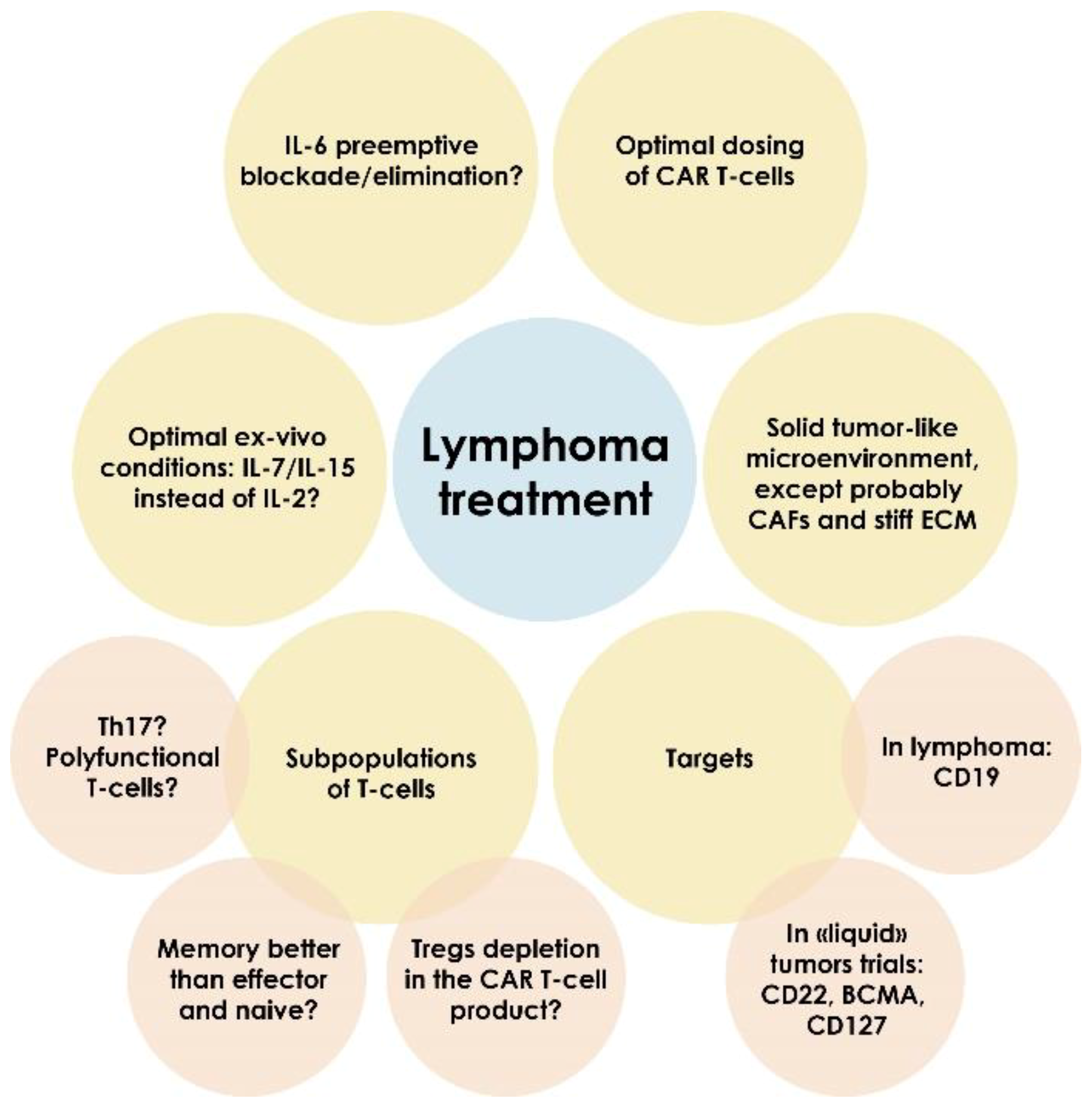
Mesothelin redirected chimeric antigen receptor t cell meso car t cell therapy has shown some efficacy in clinical trials but antitumor efficacy remains modest.
Car t cell pancreatic cancer clinical trial.
Although it has shown some remarkable results in blood tumors like leukemia solid tumors like pancreatic cancer have been more difficult to treat.
Food and drug administration.
This review summarizes the available preclinical data and highlights early phase clinical trials using car t cell approaches in pancreatic cancer a disease state that is gaining attention as a.
But the emerging form of immune treatment called car t cell therapy is gaining traction as its potential superstar.
The initial clinical trial supported by this effort will be the first trial to test a car t cell therapy designed to target a protein on cancer cells called cd33 in children and young adults with advanced forms of acute myeloid leukemia aml.
It will ultimately enroll patients at six different sites starting at the nih clinical center in.
Updated updated 28 03 2018 share.
An australian first trial of a potential new treatment for pancreatic cancer known as car t cell immunotherapy has received a 2 million funding boost.
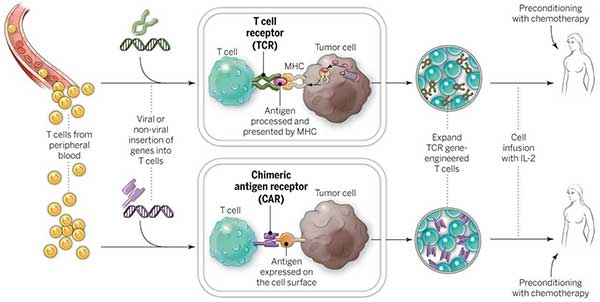
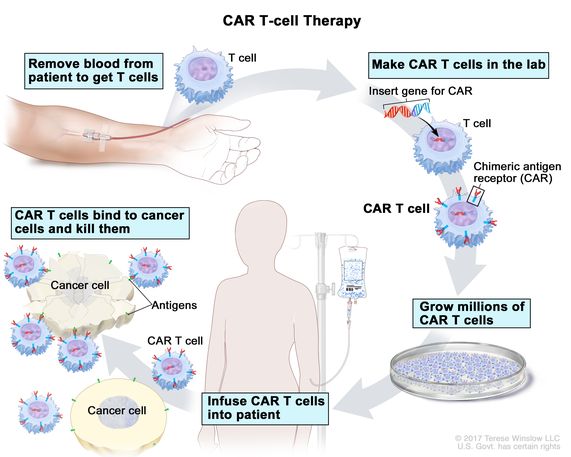
When the t cells are returned to the body they hunt down and destroy all cells with that fingerprint.
This modality utilizes genetically engineered t cells that are redirected to specific cancer associated antigens to elicit potent cytotoxic activity.
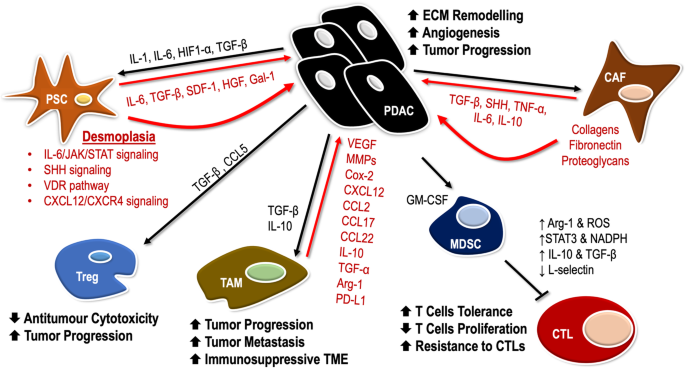
Pancreatic ductal adenocarcinoma pda is characterized by its highly immunosuppressive tumor microenvironment tme that limits t cell infiltration and induces t cell hypofunction.
Other car t cell therapies have already been tested in humans in early phase trials for a variety of cancer types but no such therapy has yet been approved by the u s.
We take some of the body s t cells a type of white blood cell and genetically retrain them to find a specific chunk of biological code the cancer fingerprint.
Car t cell immunotherapy for pancreatic cancer the safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Li and colleagues conducted a first in human open label single arm phase 1 clinical trial to evaluate autologous car cldn18 2 t cells in patients with advanced gastric or pancreatic cancer whose tumors expressed claudin 18 2.
Listing a study does not mean it has been evaluated by the u s.